No one can describe exactly what hagfish slime is like to hold. It has been compared to gelatin, but softer. To the stretchy toy putty Flarp, but wetter. Snotty egg whites is a phrase that comes to mind. “You just have to see it in person. You would be surprised,” says Douglas Fudge, a marine biologist at Chapman University in Orange, California, and one of a handful of researchers around the globe who study hagfish and their slime.

Sticky mucin and super-strong threads give hagfish slime its novel properties.
CREDIT: CHAPMAN UNIVERSITY
A bizarre eel-like creature that lives on the ocean floor, the hagfish mostly scavenges — searching out decay with its one nostril, and using its two rows of horny teeth to grab carcasses. When threatened by a fish or shark chomping down on its body, the hagfish secretes slime components from glands along its flanks, and in a matter of milliseconds, the predator finds its mouth and gills clogged with a sticky mass. It drops the hagfish and swims quickly away, hacking and writhing to clear the goo.
The slime has become a new rage in materials-science laboratories. It intrigues for many reasons: the speed at which it deploys, the nature of the two molecules (each innocuous alone) that react with seawater to form it in copious amounts, and its structure and flow. The globby pile possesses a combination of properties not seen in other biomaterials that, if harnessed in the laboratory, could spawn eco-friendly, high-performance fibers and other innovations. The US Navy is synthesizing slime threads in the lab to develop novel diver defenses, while other researchers investigate whether hagfish slime could inspire new types of foods.
Ancient slime
The hagfish is an ancient creature that has a notochord, the evolutionary precursor of a spine, and a skull made of cartilage instead of bone. It has velvety skin, not scales (think naked mole rat of the deep sea). It has changed little in the last 300 million years. About eighty species have been described all over the world, living at depths of ten meters to two thousand meters (33 to 6,500 feet). Marine biologist Bo Fernholm of the Swedish Museum of Natural History in Stockholm has seen them “sprinting up from the bottom” to feast on bait put out during shark-watching cruises off the coast of South Africa.
Because hagfish have a simple, primitive body plan with no gas-filled spaces, they can be brought from the deep and studied in labs relatively easily as long as they are kept in clean, cold seawater. Fudge’s lab has mostly studied Myxine glutinosa, the Atlantic hagfish, and Eptatretus stoutii, the Pacific hagfish. “What’s really cool is that in comparative work on the slime between just those two species, everywhere we look we see differences,” Fudge says. “We expect there’s a lot more diversity out there, we just haven’t had the chance to look yet.”
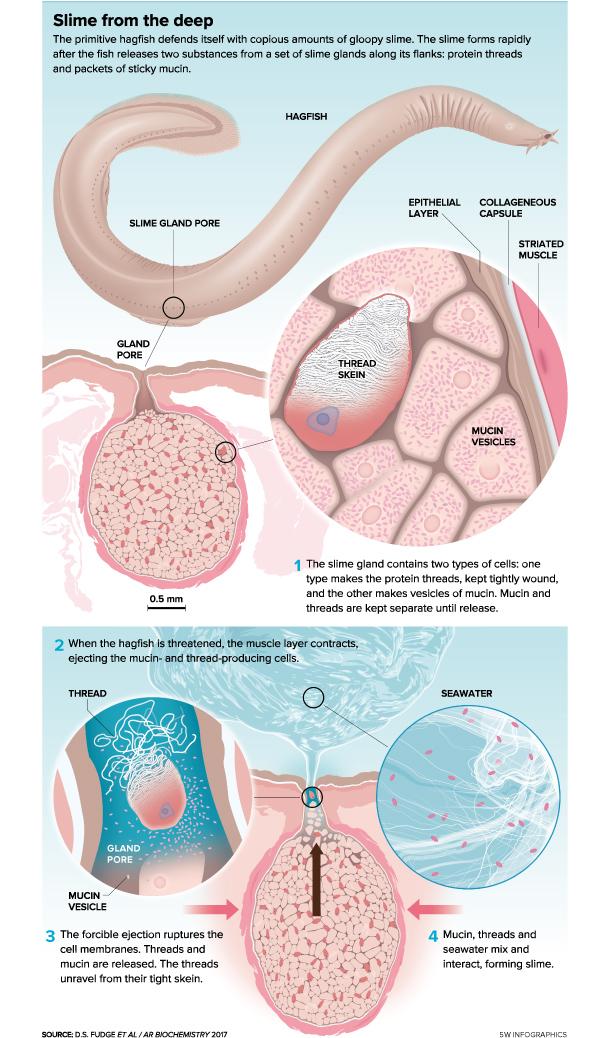
When seized by a predator, the hagfish shoots out two components from the slime glands lining its body: packets of mucin (the snotty component) and slime threads, both by the thousands. Each is made, and kept separate, inside specialized cells in the glands, but the forced ejection rips those cells’ membranes, allowing the molecules to mix with each other and surrounding seawater, forming a material known as a hydrogel. It all happens lickety-split. Grabbing a hagfish housed in a five-gallon bucket will produce a mass of slime within a fraction of a second, seemingly out of thin water.
Generally, hydrogels take minutes to hours to swell (think of the stuff used for absorption in disposable diapers), partly because water must move by osmosis into the cross-linked polymers. By contrast, the hagfish actively injects its polymers into water, which may explain its rapid formation. Just a spoonful of precursor can produce a liter of slime in less than one-tenth of a second. And when observers reach into a bucket and pick up the soft and sticky slime, they get a second surprise: It doesn’t last long. The water trapped in it will rapidly run out.
Slime, deconstructed
No other hydrogels possess this combination of soft, tough and fleeting traits: 100,000 times softer than gelatin yet resistant to cracking. Plus it morphs, marvels Lukas Böni, a graduate student studying the slime at ETH Zurich in Switzerland. “Hagfish slime can behave like a liquid or, depending on how you treat it, become almost solid,” he says.
The threads in particular have captivated researchers’ imaginations for their potential to replace high-performance fibers such as Kevlar, nylon and polyester. Specialized cells housed in the slime glands synthesize them by bundling together proteins called intermediate filaments, which form structural rods in the shape of a helix, or coiled spiral. A single cell produces one continuous thread that is about 15 cm (6 inches) long — 1,000 times the length of the cell itself.
Decades of work by a few slime enthusiasts has unraveled how the threads’ strength is produced and unleashed. Looking at thread cells under high-powered electron microscopes in the 1980s, Fernholm saw threads tightly wound inside, just like skeins of yarn for knitting, and organized in a series of overlapping figure eights, much like the ropes on a ship’s deck that quickly deploy a sail or harpoon. When threads meet water, they spring to their full length.
Three decades later, Fudge’s group used scanning and transmission electron microscopy to witness in 3-D how the specialized thread cells of the hagfish pack layer upon layer of thread around the cell’s nucleus. Work by Fudge’s lab and Böni and colleagues hints that the skein is held together by a protein glue that dissolves upon hitting seawater.

Swiss graduate student Lukas Böni studies slime for possible applications in food technology. A spoonful of precursor can produce a liter of the slime in less than one-tenth of a second.
CREDIT: SIMON KUSTER/ETH ZURICH
Though the slime’s strength is not fully understood, the fibers hold at least one key. “Hagfish slime threads get stronger as you pull on them, which is unique,” says Josh Kogot, a biochemist at the US Naval Surface Warfare Center in Panama City, Florida. As Fudge found during his PhD research, a thread will stretch up to 35% beyond its original length and then snap back. Pulled beyond that, it becomes stiffer as the intermediate-filament protein shape-shifts from a stretched-out helix to a sheet-like structure, which can form stable stacks. That gives the fiber super strength up to its breaking point, which occurs at 3.2 times its original length. In other words, a 15-cm thread can stretch to 48 cm before breaking.
Slime ahoy!
The strength is of great interest to Kogot, who heads the Navy’s biotechnology research laboratory, which looks for biomimetic solutions to solve problems for the military. Strong renewable and biodegradable threads could replace petroleum-based fibers such as nylon and polyester in everything from clothing to car-seat upholstery. But the thread’s high strength-to-weight ratio also makes it ideal for high-performance uses in objects that must be light as well as tough, such as parachutes, bicycle tires and body armor. Biological threads could theoretically act like bullet-stopping Kevlar fibers, but be made more easily, without toxic solvents or by-products.
With such applications in mind, Kogot has now reproduced slime threads in the laboratory. First he inserted hagfish genes into E. coli bacteria and turned them into factories to make the proteins. Next he used physical manipulations to get the proteins to form fibers. Kogot is now filing a patent and plans to continue developing the threads for uses such as diver, fire and ballistic protection.
Kogot’s team also wants to reproduce slime in its entirety for a wide variety of Navy applications. He imagines it might repel barnacles as an eco-friendly anti-fouling agent on ships, or act as an onboard fire-fighting solution: The slime, after all, is essentially a network of seawater. Slime might even be bottled into a spray can for Navy divers to use to dissuade sharks, just as the hagfish does.
The military also seeks new defense mechanisms that are non-lethal and non-explosive. “One of the first ideas we had for this material was for better crowd-control,” Kogot says. “Instead of firing tear gas into a crowd, you could just slime them like Ghostbusters.”
Sublime slime
The soft-yet-tough property of slime grabs the fancy of other researchers in an entirely different way: It makes them think of food. Böni has pursued hagfish-slime culinary applications in his PhD research at ETH Zurich’s Institute of Food, Nutrition and Health, where he works with a group studying deformation and flow of Newtonian liquids and soft solids — properties that are important in many foods, from milk to flour. (The project started after one of his advisers caught a BBC documentary on hagfish.) Hagfish slime has complex flow behavior because it is both viscous like honey and elastic like gelatin, Böni explains, which could give a good mouth feel when chewing and swallowing foods.
Teaming up with a Norwegian aquarium, Böni was able to bring hagfish slime back to the lab to test its properties and then find ways to modify them. “The slime is so ephemeral,” he says. “That was the idea: How can we make it stronger?”
He started by mixing the hagfish slime with other kinds of biopolymers. Mixing it with a negatively charged seaweed polymer, carrageenan, created a strong hydrogel that also held onto water better. Then he tried soy milk, a negatively charged emulsion. Before his eyes, the “soy slime” flocculated, or curdled, much as soy curdles into tofu. But unlike tofu, the soy slime needed no heat or added acid or salt to form this more solid structure.
Further examination revealed the structure was made up of two networks — the gel made of mucins and soy emulsion, reinforced by hagfish slime threads. Such networks give foods their complex textures and mouth feel, and food scientists would like to create new types of networks to, for example, make meat substitutes with the right chewiness.
The soy-slime didn’t taste great — Böni says it was blah and mushy — but it’s given him plenty of science to chew on. He wants to know why the slime’s qualities changed by adding soy milk. “It became harder, like gelatin that has set longer. And it held water more efficiently. It still behaved as one cohesive mass, but you could cut it more easily.”
And then there’s that double network. “The threads form a network without hindering themselves and breaking while they are forming,” Böni enthuses. “That’s bio-inspiring from a food technology point of view.”
For the record: The text of this article was updated on November 30 2017. It originally said that work by Böni and colleagues hints that the skein is held together by a protein glue that dissolves upon hitting seawater. The text was amended to make clear that earlier work by Fudge had reached that conclusion also.




